The defining characteristic of forages is that they contain a large portion of cell-wall material. The amount and type of plant cell-wall material determines the nutritional quality of forages.
A young plant cell has a single outer layer referred to as the primary cell wall (Fig.1). As the plant matures, a second cell wall is laid down on the inside of the cell. The secondary wall is thicker than the primary wall, giving cells tensile strength. The primary and secondary cell walls combined make up 40-80% of the forage dry matter. The main structural components of both primary and secondary walls are two complex carbohydrates called cellulose and hemicellulose. Cellulose is one of the most abundant organic materials on earth. Because higher animals cannot produce enzymes that digest cellulose, they make use of cultures of microorganisms residing in their digestive tracts. Ruminants have the most efficient system for digesting and utilizing cellulose.
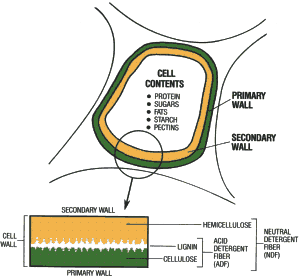
Fig 1. Diagram of a plant cell showing cell-wall structure.
With advancing maturity, forage cells insert a non-carbohydrate material known as lignin into the primary and secondary walls. This complex compound is the main constituent of wood and gives the plants additional tensile strength and rigidity. Lignin can be thought of as the primary skeleton of the plant cell. Lignin is important from a nutritional perspective because it is totally indigestible and its presence reduces the availability of the cellulose and hemicellulose portions of the forage. The primary cell wall is like a layer of bricks, the secondary wall like a layer of cinder blocks laid inside the bricks and lignin is like mortar added later between the bricks and cinder blocks. As the plant advances in maturity, more lignin is added to the complex of brick and blocks making them more difficult to break down and digest.
| Microwave method to determine moisture content of forage
Supplies: Microwave oven; small scale capable of weighing up to 150 grams (10 oz.) in small increments (2-5 grams; 0.1 oz. or smaller); dry, dinner-size paper plate; glass of water. Method: Select a representative sample of forage from all over the field. Samples should be taken from top, bottom and middle of swath. Weigh the empty paper plate and record the weight on the edge of the plate. Weigh exactly 100 grams of forage onto the plate on the scale, allowing for the weight of the plate. (For example, if the plate weighs 30 grams, the total weight of the plate and forage is 130 grams.) For US measurements use exactly 10 oz. of sample plus weight of plate. Spread the sample evenly over the plate and place it in the microwave with a half-filled glass of water in the back corner. Heat the sample for four minutes at full power. Weigh and record the weight, then stir the forage and place the plate back in the microwave for another minute, taking care not to lose any of the sample. Heat at full power but for only 30 seconds before weighing. Repeat the procedure until weight becomes constant. If the forage starts to char, shorten the drying intervals. The final constant weight, minus the weight of the plate, is the dry matter content of the forage as a percentage. (For US measurements, multiply the final weight, minus plate, by 10 to get percentage dry matter.) |
Taking the sample
Accurate results are dependent on obtaining a representative sample, proper handling of the samples after collection and good analytical procedures in the laboratory.
Sensory appraisal
Forages can be evaluated by sight, smell and feel. Useful sensory clues include: colour, leaf content, stem texture, maturity, contamination by weeds, mold or soil and observations on palatability.
Dry matter determination
Dry matter is the percentage of the forage that is not water. Dry matter content must be known to compare yield of different forages and dry matter content affects feed intake. Dry matter content also determines how forages will preserve when stored as hay and silage.
What is ‘Detergent’ Fibre?
The detergent method for assessing quality of forages was developed in the 1960’s. The earlier crude fibre system failed to generate accurate estimates of digestible nutrients over a wide range of forages; it tended to underestimate good quality forages and overestimate poor quality forages. The detergent system of forage analysis is now the most common way to assess forage quality. Fig. 2 shows a schematic of the detergent system of forage analysis. Detergent analyses are performed on dried and finely ground samples.
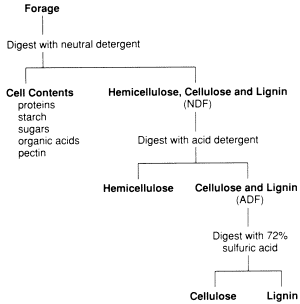
Fig 2. The detergent (Van Soest) procedure to partition forage fractions.
| Table 1. Classification of forage fractions using the Van Soest method. | ||
| Fraction | Components Included | Digestibility |
| Cell Contents | Sugars, starch, pectin, | Complete |
| Soluble carbohydrates | Complete | |
| Protein, Non-protein N | High | |
| Lipids (fats) | High | |
| Water soluble vitamins minerals | ||
| Cell Wall (NDF) | Hemicellulose | Partial |
| Cellulose | Partial | |
| Heat damaged protein | Indigestible | |
| Lignin | Indigestible | |
| Silica | Indigestible | |
Neutral Detergent Fibre (NDF)
For determining NDF, samples are boiled in a special detergent at a neutral pH of 7.0, then filtered. The soluble portion that passes through the filter contains the highly digestible nutrients contained within the cells (see Table 1).
The insoluble portion of the forage that does not pass through the filter is called the neutral detergent fibre. This fraction contains the cell-wall material including cellulose, hemicellulose, lignin and silica (Table 1). NDF fraction increases with the advancing maturity of forages.
Neutral detergent fibre is used as a negative indicator of feed intake. As the NDF increases, animals are able to consume less forage. An approximate relationship between NDF and intake is:
Feed intake (dry matter) as percent of body weight = 120/NDF%
Example: a forage with an NDF value of 40% will be consumed at 120/40=3% of body weight.
Acid Detergent Fibre (ADF)
Acid detergent fibre is the portion of the forage that remains on the filter after the forage sample is treated with a detergent and strong acid. It includes the largely digestible cellulose, indigestible lignin and inorganic silica.
Acid detergent fibre is important because it is negatively correlated with digestibility of forages. As the ADF increases, the forage becomes less digestible. ADF is the most commonly used indicator of forage quality.
Total digestible nutrient (TDN) values are calculated directly from ADF values. Note that the relationship between TDN and ADF is affected by crop type and that different labs use different equations. Typical equations are shown below:
Legumes and grasses: TDN=88.9-(0.79 x ADF%)
Corn silage: TDN=87.84-(0.70 x ADF%)
Relative Feed Value
Relative feed value (RFV) is often reported for alfalfa hay sold in the US. RFV combines estimates of dry matter intake (from NDF) with TDN (from ADF). RFV is a relative measurement to help farmers compare feeds. High-quality alfalfa and corn typically have RFV values greater than 130 and may reach over 160. Grasses rarely have values over 120. Does this mean that grasses are inferior feeds or should RFV be used only within forage class?
Lignin and silica
Lignin is the wood-like, non-carbohydrate component that cannot be digested by ruminants. Further, lignin decreases availability of cellulose, hemicellulose and protein. The lignin fraction can be determined by further treatment of the ADF fraction with a very strong acid. Some grasses accumulate large quantities of silica (or sand). The silica fraction is left as ash after a forage sample is ignited in a special furnace.
Sources of protein: microbial and rumen-bypass
All animals need to consume protein to supply the amino acids that are used to build the proteins in muscle, membranes, enzymes and milk. Ruminant animals are different from non-ruminants in how they obtain amino acids. Non-ruminants derive all their amino acids directly from the protein in their feed. Ruminants derive amino acids from two sources: from the microbes that grow in the rumen and are carried to the intestines and from the protein that passes through the rumen; both protein sources are digested in the intestines (Fig. 3). What happens to protein in the rumen?
Protein molecules are broken down by microbes in the rumen into both amino acids and non-protein nitrogen compounds such as ammonia. Rumen microbes feed on both types of nitrogen compounds. The microbes obtain their energy needs from the carbohydrates (sugars, starches, hemicellulose and cellulose) in the forage. The microbial cells that pass out of the rumen and that are digested in the intestines provide about half of the amino acids in high-producing dairy cows. Factors that favour growth of microbes in the rumen also favour amino acid supply to the cow.
Rumen microbes grow best when the supply of energy and protein or nitrogen is synchronized. Slowly digested carbohydrates such as cellulose are most compatible with protein sources having slow rates of degradation that provide a steady supply of nitrogen. Frequent meals also help to provide a steady supply of nitrogen for the microbes.
Highly digestible, immature forages supply the most rapidly digestible carbohydrates and the most readily available energy for microbial growth. With rapid digestion, feed spends less time in the rumen, freeing space for more feed intake. Rapid passage of feed through the rumen also moves more rumen microbes into the intestines.
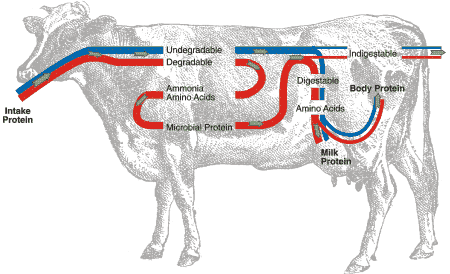
Fig 3. Schematic representation of protein digestion and utilization in the cow.
How do forages influence protein supply to ruminants?
Forages make up as much as half of the feed rations of lactating cows and therefore play an important role in supplying amino acids. Coincidentally, forages stimulate chewing and rumination, which promotes the production of up to 150 litres (40 gal.) of saliva a day. Saliva buffers the rumen environment for the microbes.
The proteins in forages contain both degradable and undegradable fractions (Fig. 4). The overall degradability of protein is determined by two factors: portion of protein that is digested in the rumen and the speed of digestion in the rumen relative to rate of passage out of the rumen. The degradable fraction can be subdivided into a rapidly degradable soluble fraction and a slowly degradable insoluble fraction.
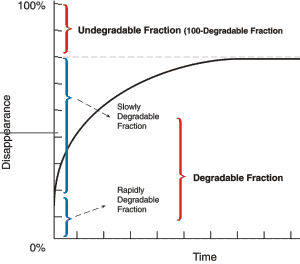
Fig 4. Disappearance of protein fractions in forages as a function of time.
A study conducted at the University of British Columbia compared the protein attributes of typical feeds used on dairy farms in coastal BC (Table 2). Surprisingly wide differences in protein degradation were found in each forage type. For example, the slowly degradable fractions in alfalfa hays (from Central Washington) ranged from 17 to 45% (average 33%) and in grass hays ranged from 25 to 63% (average 46%). The slowly degradable fraction in alfalfa was generally digested faster than that in local grass hays. These results show that at present we cannot predict the exact protein characteristics in local forages in order to formulate precise rations.
| Table 2. Ratio of rapidly to slowly degradable fractions in forage classes used in coastal B.C. | |
| Alfalfa Hay | 2:1 |
| Grass Hay | 1:1 |
| Grass Silage | 1:1 |
| Corn Silage | 3:1 |
| (Adapted from Von Keyserlingk, 1994. Ph.D. Thesis, Univ. of BC.) | |
Factors which affect the rate of protein breakdown in forages
Feeds pass very rapidly through the rumen of high-producing dairy cows consuming large amounts of feed. Hence, rumen microbes have little time to digest the protein that they require for their own growth. It is important to understand the factors that affect the rate of breakdown of protein in the rumen.
Growing conditions, level of nitrogen fertilization, maturity, time of year and conservation method influence the rumen degradability of forage protein. High rates of nitrogen fertilization decrease the undegradable fraction, hence increase degradability. Increasing stage of maturity and progression of the growing season decrease protein degradability. During the ensiling and drying processes, protein can be damaged by heat, decreasing the soluble fraction and increasing the undegradable fraction.
Grass hay often contains more soluble protein than fresh forage because some insoluble protein is converted to a soluble form during drying. However, hay has a lower protein degradation rate and a larger undegradable fraction than fresh forage, which ultimately results in overall less effective degradation.
During ensiling, enzymes released from collapsing plant cells digest protein into peptides and amino acids. Bacteria further digest the peptides and amino acids into simpler molecules such as amines and ammonia. At the same time, the bacteria consume the sugars in the forages and release acids that have low energy value for rumen microbes. Therefore, ensiled grass usually has a poor balance between available energy and nitrogen for rumen microbes. The changes to protein during ensiling reduce the value of silage protein so that protein supplements must be provided.
When forages are wilted prior to ensiling, the water loss concentrates the salts in the cell solution, which tends to reduce the fermentation of the protein in the silage. Protein breakdown in silage can also be reduced by additives such as formic acid, formaldehyde and microbial inoculants. Formic acid quickly reduces pH and stabilizes the silage, but has no influence on the protein degradation in the rumen. Formaldehyde has a dual action; it kills destructive anaerobic bacteria called ‘Clostridia’ and decreases protein degradation in the silage and in the rumen by bonding to the proteins.
The Faculty of Agricultural Sciences at UBC has been investigating novel methods for reducing breakdown of protein during ensiling. The research has revealed that protein degradation during ensiling can be reduced by applying so called ‘masking agents’ that reduce the activity of the protein-destroying enzymes.
Reduction of the degradation of protein in the rumen is beneficial only if the protein escaping degradation in the rumen is digested in the intestines. Forage proteins associated with cell walls escape digestion in the rumen but cannot be digested in the small intestine, and are only slightly digested in the large intestine.
Recent work at the University of British Columbia showed that only 20% of the protein produced by a grass crop stored as silage is absorbed in the small intestines; intestinal absorption of ensiled corn protein is only 10%.
Because so much of the forage protein is digested in the rumen, the composition of amino acids entering the small intestines differs greatly from the composition of the consumed forage. There are also wide differences among forages in how easily amino acids can be absorbed in the intestines, but there are no clear differences among fresh, dried and ensiled forages. The exact requirements of amino acids are still not known. Interestingly, when dairy cows are given diets containing a high proportion of grass silage, the source of supplemental protein has a great influence on milk production.
Conclusion
Current thought is that ruminants have specific requirements for amino acids rather than for certain proteins. However, evaluating protein sources and formulating rations still requires characterization of proteins in terms of their degradability in the rumen. The ultimate goal of new feed models is to formulate rations that meet the precise nitrogen needs of rumen microbes as well as the specific amino acid requirements for each class of ruminant animal.
Contributed by J. Baah and J. A. Shelford, Faculty of Agricultural Sciences, University of British Columbia
Harvesting forages as silage is a compromise between minimizing field and fermentation losses. Efficient fermentation ensures a more palatable and digestible feed, encouraging optimal dry matter intake that translates into improved animal performance. The primary management factors that are under the control of the producer are:
1. Stage of maturity of the forage at harvest.
2. Type of fermentation that occurs in the silo or bunker.
3. Method of harvesting, type of storage structure, silo management and method of feeding.
Attention to details such as speed of harvesting, moisture content, length of chop, silage distribution and compaction can improve the fermentation process and reduce storage losses.
Six Phases of the Ensiling Process
PHASE 1 Aerobic microorganisms are present on the forage surface at the time of harvesting. Aerobic respiration by freshly cut plant material and aerobic bacteria begins at harvesting and continues after the forage is piled and packed. Aerobic respiration consumes the oxygen contained within and between the forage particles creating the desired anaerobic conditions. Aerobic respiration also consumes the soluble carbohydrates needed by the beneficial lactic acid bacteria and by rumen microbes.
Phase 1 ends when all the oxygen has been consumed. Under ideal conditions, Phase 1 lasts only a few hours; with improper management, this phase may continue for several weeks and result in significant reduction in feed quality.
The respiration process produces water and heat in the silage mass. Excessive heat build-up during Phase 1 can greatly reduce the digestibility of proteins. Plant enzymes break down proteins during Phase 1. Proteins are first reduced to amino acids and then to amines and finally to ammonia. Up to half of all the plant protein may be broken down during this process. As the silage becomes more acidic, the activity of these enzymes declines.
Good silage-making technique minimizes air infiltration to shorten the time required to achieve an anaerobic environment. Key management factors are crop choice, content of soluble carbohydrate, crop maturity, moisture content, chop length, rate of filling and packing, and proper sealing of the storage structure.
| Table 3. Average nutrient composition of farm-grown forages from the South-Coastal Forage Competition in BC in the years 1993-97. (Courtesy of D. Bates, BC Ministry of Agriculture and Food.) | ||||||
| Nutrient | Grass Hay | Grass Silage | ||||
| Low | Avg | High | Low | Avg | High | |
| Dry matter (%) | 80.6 | 88.1 | 91.8 | 19.5 | 40.2 | 75.1 |
| Acid Detergent Fiber (%) | 23.8 | 29.8 | 38.1 | 23.4 | 31.2 | 41.7 |
| Neutral Detergent Fibre (%) | 43.9 | 56.3 | 65.9 | 33.3 | 48.9 | 65.3 |
| Total Digestible Nutrients (%) | 55.8 | 66.8 | 74.6 | 50.8 | 65.0 | 75.4 |
| Crude Protein (%) | 9.1 | 17.6 | 24.4 | 8.8 | 17.7 | 26.1 |
| Nitrogen (%) | 1.5 | 2.8 | 3.9 | 1.4 | 2.8 | 4.2 |
| Nitrate-N (%) | 0.01 | 0.11 | 0.40 | 0.0 | 0.05 | 0.26 |
| Ammonium-N as % of total N | 3.6 | 17.5 | 47.5 | |||
| Heat Damaged Protein (%) | 2.4 | 4.4 | 11.2 | |||
| pH | 3.9 | 4.9 | 6.4 | |||
| Phosphorus (%) | 0.19 | 0.33 | 0.48 | 0.22 | 0.37 | 0.55 |
| Potassium (%) | 1.17 | 3.13 | 4.99 | 1.30 | 3.05 | 4.50 |
| Magnesium (%) | 0.13 | 0.23 | 0.38 | 0.11 | 0.24 | 0.53 |
| Calcium (%) | 0.26 | 0.47 | 0.85 | 0.28 | 0.56 | 1.39 |
PHASE 2 Phase 2 begins when anaerobic bacteria take over. These bacteria ferment soluble carbohydrates into acetic acid. Acetic acid production is desirable because it reduces pH to set up the succeeding fermentation phases. Also, acetic acid can be used as an energy source by rumen microbes. Phase 2 usually lasts no longer then 24 – 72 hours, ending when the pH of the ensiled mass falls below 5.0, killing the acetic acid-producing bacteria.
PHASE 3 This is a transition phase in which the lower pH favours the growth of an anaerobic group of bacteria that produce lactic acid, replacing those that produce acetic acid.
PHASE 4 In this Phase the lactic acid bacteria predominate. Lactic acid is the most desirable of the fermentation acids. In well-preserved silage, lactic acid should comprise more than 60% of the total silage organic acids and the silage should contain up to 6% lactic acid on a dry matter basis. Lactic acid can be utilized by cattle as an energy source. Phase 4 is the longest phase in the ensiling process as it continues until the pH of the forage is low enough to inhibit the growth of all bacteria. When this pH is reached, the forage is in a stable state so long as oxygen is excluded.
| Table 4. Effect of adding absorbent feedstuffs to direct-cut grass silage on effluent and content of water-soluble carbohydrates and crude protein, relative to wilted silage. | |||
| Additives | Effluent | Water soluble carbohydrates | Crude protein |
| % | % | % | |
| Wilted silage (28% dry matter) | 4.5 | 13.9 | 22.1 |
| Direct cut silage (16% dry matter) | 18.0 | 12.8 | 22.5 |
| + 10% Barley | 11.8 | 16.8 | 20.7 |
| + 10% Beet pulp | 9.2 | 23.0 | 20.5 |
| + 10% Alfalfa cubes | 6.3 | 12.2 | 22.3 |
| + 20% Alfalfa cubes | 1.9 | 14.9 | 21.3 |
| + 30% Alfalfa cubes | 0.6 | 16.0 | 22.3 |
| (based on S. Fransen and F. Strubi. 1998. J. Dairy Sci. 81: 2633-2644) | |||
PHASE 5 The final pH of the ensiled forage depends largely on the type of forage being ensiled and the condition, especially moisture content, at the time of ensiling. Haylage should reach a final pH of around 4.5 and corn silage near 4.0. Drier silage generally has higher stable pH than wet silage. The pH alone is not a good indicator of the quality of silage or of the type of fermentation that occurred.
Forages ensiled at moisture levels greater than 70% may undergo a different version of Phase 4 where undesirable Clostridia bacteria proliferate instead of lactic acid bacteria. Clostridia bacteria produce butyric acid rather that lactic acid, which results in sour silage. With this type of fermentation the pH may stabilize at 5.0 or above.
PHASE 6 This phase refers to the silage as it is being fed out from the storage structure. This phase is important because up to 50% of the silage dry matter losses occur from secondary aerobic decomposition. Phase 6 occurs on any surface of the silage that is exposed to oxygen while in storage and in the feed-bunk. High populations of yeast and mould can lead to significant losses due to aerobic deterioration of the silage. Proper management is vital to reduce these losses and improve the bunk-life (aerobic stability) of the silage.
Silage effluent represents a loss of valuable forage nutrients. Effluent is also a potent environmental pollutant that poses a threat to fish habitat because of its very high biological oxygen demand (BOD) and high concentrations of soluble protein and ammonia. The best way to minimize effluent from silage is to wilt the crop prior to harvesting, but poor weather when the crop is ready for harvest may make wilting impossible. Under these conditions, absorbents may be added to silage to reduce effluent.
A study conducted by Washington State University at Puyallup compared the effectiveness of different absorbents in reducing effluent from direct-cut silage. There was little effluent when the dry matter content of the silage was above 27%, but below 20% dry matter effluent loss ranged from 10 – 20% of the dry matter (see Table 4). The study showed that adding 10% barley, beet pulp, or alfalfa cubes successfully reduced effluent, but alfalfa cubes were the most effective of the three additives at equivalent weight. In terms of effect on nutritional quality, beet pulp raised the concentration of water-soluble carbohydrates most but alfalfa cubes increased crude protein the most. Cost of additives was not considered in this study.
|
The Fescue Endophyte Story Tall fescue rapidly became the most important cultivated forage grass in the US after its introduction in 1931. Farmers quickly recognized that tall fescue is well adapted to a wide range of soil and weather conditions and offers a considerable advantage in yield over many other grasses. Unfortunately, several health and performance problems were reported in stock feeding on tall fescue. These included low feed intake, low weight gains, poor reproductive performance, lower milk production and higher body temperature. The syndrome had a number of names including fescue toxicosis and summer syndrome. The reasons were not apparent from chemical analysis of the feed. The first clue to the cause of fescue problems came in 1976 when researchers in the state of Georgia discovered that problem pastures were heavily infected with a fungus living in the fescue plants whereas uninfected pastures were free of animal health problems. The uninfected pastures had been inadvertently established with old seed in which the fungus had died before planting. Numerous trials have since shown that the fungus causes low feed intake, poor animal gains, low conception rates and poor milk production. The offending fungus is referred to as an endophyte because it lives within the plant without parasitizing or harming it. The fungus cannot be seen with the unaided eye but can easily be detected in a laboratory. Curiously, the fungus offers infected plants some protection against insects, diseases, and even environmental stresses such as drought. While forage seed producers are working to eliminate endophyte from their seed, turf producers are putting endophyte back in to take advantage of this protection. The fescue endophyte fungus goes by the name Acremonium coenophialum. The endophyte is now known to be transmitted only by seed so it cannot spread across fields or even from plant to plant. A new stand of tall fescue planted with clean seed will not contain any endophyte-infected plants. All certified tall fescue seed is now endophyte-free. |
Spring maturity of grass varieties adapted to coastal BC and the Pacific Northwest ranges by as much as 3 – 4 weeks based on comparable growth stages. Therefore, producers can plant a set of varieties with contrasting maturities so that they will be able to harvest all crops at the appropriate growth stage. Having a range of maturities is also a hedge against a stretch of bad weather.
A study at PARC (Agassiz) compared early- and late-maturing varieties of orchardgrass, tall fescue and perennial ryegrass in terms of yield and nutritional quality.
When harvested on the same day, the late maturing varieties of orchardgrass yielded 10 – 20% less in first cut than early varieties. On the other hand, when the early and late varieties were harvested at the same growth stage, say ‘late boot’, their annual yield was similar. Although annual yield was similar, seasonal distribution of yield was not. First harvest taken at the boot stage yielded 25% of annual production for the early variety, but over 40% of annual production for the late variety. Note that this means more eggs in one basket for the late variety, which partly offsets the advantage of the hedge against poor weather mentioned above. Research at PARC (Agassiz) has shown that the late varieties require more fertilizer for first cut than early varieties (see Ch. 3).
| Table 5. Yield and quality of early (Hallmark) and late (Mobit) orchardgrass varieties harvested at the ‘boot’ growth stage. Hallmark matures about three weeks before Mobite. | ||
| Early Maturing |
Late Maturing |
|
| Yield | t/ha of dry matter * | |
| Annual yield | 14.0 | 13.5 |
| First harvest yield | 3.5 | 5.4 |
| First harvest as % of annual yield | 25% | 40% |
| Quality of First Cut | % | |
| Crude protein | 17.0 | 11.0 |
| Neutral detergent fibre | 58.2 | 60.9 |
| Acid detergent fibre | 30.5 | 33.5 |
| Lignin | 3.1 | 4.2 |
| *for T/ac multiply by 0.45 | ||
As expected, when compared on the same calendar date, the nutritional quality of the late variety was much better than the early variety. Surprisingly, early varieties had better nutritional quality than late varieties when compared at the same growth stage. For example, at the boot stage, the late orchardgrass had 3% more ADF, 2.5% more NDF, 1.1% more lignin, and 3-6% less crude protein than the early variety.
The same trend was observed for perennial ryegrass and tall fescue. Perennial ryegrass has a maturity range of up to four weeks while tall fescue has a range of up to three weeks.
What do these results mean for selecting varieties and scheduling harvests? Indeed, use late-maturing varieties to spread out the harvest season- loss in yield, if any, will be small. You may delay harvesting the late varieties but not as long as indicated by their growth stage. For a late variety that matures two weeks later than an early one, harvest only one week later to ensure comparable quality. Note that late varieties should receive a greater proportion of the annual fertilizer allocation for the first harvest compared to early varieties.
The long growing season, moderate temperatures, high humidity and mild winters support the growth and survival of pathogenic organisms in coastal BC and the Pacific Northwest. The most important leaf disease in the region is stripe rust in orchardgrass. As the name suggests, stripe rust pustules are arranged in characteristic stripes on the leaves. Outbreaks of stripe rust strike south coastal BC in late summer and fall.
Stripe rust overwinters in BC only in the very mild years. More commonly, it blows in from southern Oregon and northern California on southerly summer winds. Once established, the pathogen prefers warm dry days with 2-3 hours of morning dew on the leaves. Under these favourable conditions the stripe rust fungi can double in number every 4-5 days.
Stripe rust reduces digestibility of orchardgrass and increases both acid (ADF) and neutral detergent fibre (NDF) values. There is a direct correlation between visual severity of the disease and increase in ADF concentration (Fig. 5). Effect on protein content is less pronounced.

Fig 6. Variety differences in resistance to stripe rust.
Farmers can minimize the impact of stripe rust by planting resistant varieties of orchardgrass (contact supplier), planting some land to tall fescue, providing ample fertilizer and water and harvesting soon after the disease appears.
Other fungal diseases are thought to have a similar effect on quality although there is less direct evidence. Tall fescue and perennial ryegrass are resistant to stripe rust but both grasses are susceptible to crown rust and stem rust. These diseases tend to proliferate earlier in the season than stripe rust.
Leaf scald overwinters locally and is favoured by cool humid conditions in spring and early summer. The disease starts with scald-like lesions on leaves that spread and kill off large segments turning them brown.
